23
2023
-
03
Modeling and Experimental Study of Ore-Carbon Briquette Reduction under CO–CO2 Atmosphere (1)
Keywords:
ore-carbon briquette,CO–CO2 atmosphere,reduction, simulation, re-oxidation
Source: internal company
Abstract
1. Introduction
An ore-carbon briquette is a composite briquette consisting of iron-bearing oxide and carbonaceous materials that were used as feed material in some coal-based direct reduction processes, such as FASTMET® (FASTMET is a trade mark of MIDREX Co., USA.) and ITMK3® (ITMK3 is a trademark of KOBE Steel Co., Japan.) . The use of these briquettes offers advantages, such as a high reduction rate, utilization of non-coking coal, and biochar for producing sponge iron economically. The ore-carbon briquette reduction technology is often used in treating various metallurgical dust and sludge recovering valuable metals, such as nickel and titanium, from complicated minerals, and upgrading refractory iron ores by removing detrimental minerals, such as quartz and alumina. The reduction of ore-carbon briquettes is usually carried out at the industrial level using rotary hearth furnace (RHF) reduction technology. In an RHF process, the briquette is fed onto the rotating hearth of the RHF and is reduced into sponge iron in a high-temperature environment. The flue gas from the combustion of coal gas, composed of CO, CO2, H2, and H2O, forms a weakly oxidative atmosphere on the surface of the briquette. The CO2 or H2O levels may allow for the metallic iron oxidation of the ore-carbon briquette during the RHF process, which would have a negative effect on the quality of the products. Therefore, studies are required to address the reduction behavior of ore-carbon briquettes under the RHF reactive atmosphere.
The reaction kinetics of iron ore-carbon briquettes under inert atmosphere (nitrogen or argon) have been extensively studied, and their major features are well established. The reduction of the briquette proceeds rapidly under high temperatures and generates a large amount of gas inside the briquette, making it more complicated than the reaction behavior of an iron ore or coal briquette. The reaction kinetics depend significantly on the chemical composition and physical properties of the briquette. Some studies on ore-carbon reduction have been conducted under the oxidative atmosphere by Singh et al. and by Ghosh et al., but such studies are scarce. There are several mathematical descriptions of the reduction phenomenon of the iron ore-carbon briquette. For example, Moon et al. developed a model with an assumption of uniform conversion of iron oxide and carbon particles in the briquette, Sun and Lu and Shi et al. developed models including the expressions of chemical kinetics, equations of mass transfer, and equations of heat transfer, and Donskoi et al. developed a model when considering the swelling/shrinkage of the briquette. Although these models attempt to give a comprehensive understanding of the reduction behavior of the briquette, the interaction between the briquette and the oxidative atmosphere has not been included. Therefore, the effect of the oxidative atmosphere on the reduction behavior of ore-carbon briquette has been overlooked in the existing studies.
2. Experiments
2.1. Materials and Briquette Preparation
| Fe2O3 | SiO2 | CaO | Al2O3 | MgO | MnO | LOI |
| 91.77 | 2.9 | 0.1 | 4.05 | 0.56 | 0.14 | 0.48 |
|
LOI: loss on ignition.
|
||||||
| Volatile | Fixed Carbon | Ash |
| 0.41 | 96.3 | 3.29 |
2.2. Experimental Setup and Procedures
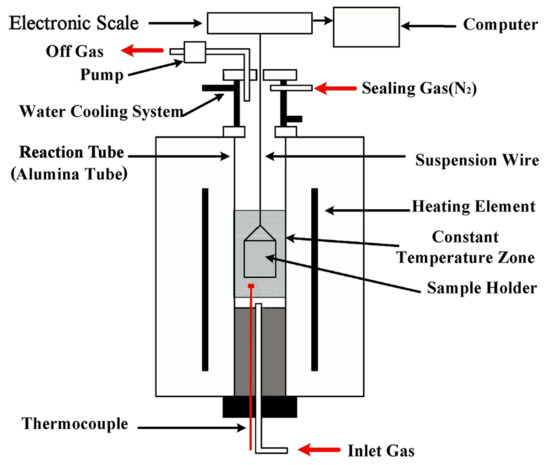
The furnace was preheated to the desired temperature under N2. One briquette was then loaded into the sample holder, preheated at 773 K for 5 min in the upper part of the reaction tube, and then introduced into the hot zone. At this time, the N2 feed was replaced with a CO–CO2 gas mixture, PCO/PCO2 = 1.0, and the mass loss/gain of the briquette was measured by an electronic scale and was recorded by a computer at an interval of 2 s. After the predetermined time, the briquette was withdrawn from the reaction tube and quenched by a N2 stream. In all of the individual tests, a constant gas flow rate of 1600 cm3/min (Standard Temperature and Pressure) at the gas inlet was maintained. In addition, some reduced briquettes were subjected to carbon content analysis, scanning electron microscopy (SEM), and energy dispersive spectrometry (EDS) examinations. The carbon analysis was conducted using a CS-2800 infrared carbon sulfur analyzer (NCS, Beijing, China); SEM and EDS were performed using a Quanta-250 scanning electron microscope (FEI, Hillsboro, OR, USA). The defifinitions and calculation methods of mass-loss fraction (fm), reduction degree (fo) and carbon conversion (fc) of the tested briquette are fm = ∆mt/(mC + mO), fc = ∆mC/mC = 1.0 − (mb − ∆mt)[C]t/mC, and fo = ∆mO/mO = fm + mC/mO(fm − fc), respectively [30], where, ∆mt , ∆mC, and ∆mO are the mass loss, carbon mass loss, and oxygen mass loss of the briquette at time t, respectively; mb , mC,and mO are the initial mass, initial carbon mass, and initial iron-oxide oxygen mass of the preheatedbriquette, respectively; and [C]t is the carbon content of the sample at time t. mb , mC, and mO areavailable, according to the preparation procedure of the briquette.
3. Mathematical Model
The overall reduction process is believed to occur through an intermediate gas phase. The reactions that take place in the briquette are the stage-wise reductions of iron oxide by CO (Equations (1)–(3)), the Boudouard reaction of carbon particles (Equation (4)), and metallic iron oxidation (Equation (5)). The reduction of iron oxide by solid carbon may also occur; however, it is difficult to estimate the extent of this reaction, and the share of the solid-state reduction is minimal when considering the much larger gas–solid contact area. Therefore, no solid–solid reaction was taken into account.
Fe3O4(s) + CO(g) = 3 FeO(s) + CO2(g) (2)
FeO(s) + CO(g) = Fe(s) + CO2(g) (3)
C(s) + CO2(g) = 2CO(g) (4)
Fe(s) + CO2(g) = FeO(s) + CO(g) (5)
The gas phase in the briquette was considered to be ideal and to consist of CO and CO2.






Related news
2024-06-13

